Abstract
Introduction
Prognosis for relapsed and refractory large B-cell lymphoma (r/r LBCL) is poor, and treatment remains challenging. Relmacabtagene autoleucel (relma-cel) is a CD19-directed 4-1BB/CD3z chimeric antigen receptor T cell (CAR-T) product manufactured in China. RELIANCE study is the first CAR-T study with CD19 as target that got IND approved by the authority in China. Initial findings of the pivotal Phase 2 single-arm, multicenter RELIANCE trial demonstrated high response rates and low rates of CAR-T-associated toxicity with relma-cel treatment in heavily pretreated patients (pts) with r/r LBCL (NCT04089215; Ying et al. Cancer Med 2020;10:999-1011). Long-term follow-up data for the RELIANCE study are presented.
Materials & Methods
Fifty-nine adults (age ≥18 years) with heavily pretreated (≥2 prior therapies), measurable and histologically confirmed r/r LBCL were randomized to receive lymphodepletion chemotherapy (LDC: fludarabine 25 mg/m 2 + cyclophosphamide 250 mg/m 2 daily×3) followed by a single infusion of autologous CAR+ T cells at a dose of 100×10 6 or 150×10 6. Pts were evaluated for efficacy using Lugano criteria and for toxicity using Common Terminology Criteria for Adverse Events v4.03 and for cytokine release syndrome (CRS) using Lee et al. (Blood 2014;124(2):188-95). Primary endpoint was 3-month objective response rate (ORR); key secondary endpoints included complete response rate (CRR) at 3 months, duration of response (DOR), progression-free survival (PFS), overall survival (OS) and treatment-emergent adverse event (TEAE) profile. The data cutoff date was December 31, 2020.
Results
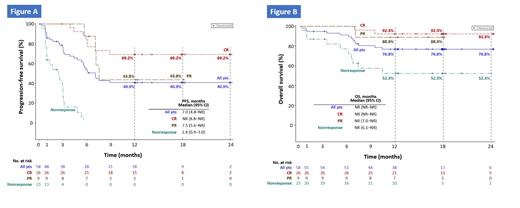
At the time of analysis, all 59 pts (median age 56.0 years, range 18-75 years) were at least 15 months post-treatment with relma-cel (7 pts completed trial, 34 pts on study, 18 pts withdrew). Among the modified intent-to-treat (mITT) population of 58 efficacy-evaluable pts, best ORR was 77.6%, with a best CRR of 51.7%; ORR and CRR at 3-month-postdose landmark were 60.3% and 44.8%, respectively. With a median follow-up of 17.9 months (range 0.3-25.6 months), median PFS (mITT) was 7.0 months (95% CI 4.8-not reached [NR]). Median PFS was NR (95% CI 8.8 months-NR) for pts with compete response (CR) at 3 months, the 6-, 12-, 18- and 24-month PFS rates were 80.8%, 69.2%, 69.2% and 69.2%, respectively (Figure). PFS was associated with objective response or CR at 1, 3, 6, and 12 months (log-rank test, p≤0.002). Median OS was NR (95% CI NR-NR) for both the mITT population and pts with CR at 3 months (12-month OS rate, 76.8% and 92.3%, respectively). Median DOR was NR (95% CI 4.9 months-NR) for the mITT population and (95% CI 8.0 months-NR) for pts with CR at 3 months. Levels of CAR-T cells declined to below the level of quantification (BLQ) in 41 (69.5%) pts, among whom nearly 40% had sustained response. Median time from documentation of CAR-T BLQ to disease progression was 6.1 months (95% CI 1.8-NR). Treatment-related TEAEs were reported in 93.2% of pts and were primarily grade 1-2 (89.8% pts); 54.2% of pts had grade ≥3 TEAEs. Pyrexia was the most common TEAE, reported in 59.3% of pts (all grade 1-2). The most common grade ≥3 treatment-related TEAEs were neutrophil count decrease (30.5%) and white blood cell (WBC) count decrease (13.6%). Presence of cytopenia at day 29 (78.0%) was significantly associated with WBC count after LDC, change in serum albumin between days 1 and 4 and serum interleukin 8 after LDC (p<0.05). The incidence of CRS and neurotoxicity (NT) was 47.5% and 20.3%, respectively, both primarily grade 1-2. There were no CRS- or NT-related deaths. Occurrence of grade ≥2 CRS or NT (11.9% and 6.8%, respectively) was significantly associated with fold-change of platelet count on day 4 compared with baseline and day 1 absolute neutrophil count (p<0.03). There were no treatment-related deaths.
Conclusions
These are the first data of long-term follow-up CD19 CAR-T study with IND approval in China reported. With nearly 18 months of median follow-up, the RELIANCE pivotal registered study demonstrated durable responses, high rates of OS and PFS, and no new safety profile found in r/r LBCL pts. Based on these data, relma-cel is undergoing approval in China. Figure: Kaplan-Meier estimates of (A) PFS and (B) OS by 3-month tumor response
PR, partial response
Zhang: JW Therapeutics: Current Employment. Yang: JW Therapeutics: Current Employment. Zhou: JW Therapeutics: Current Employment. Zheng: JW Therapeutics: Current Employment.